Introduction: Extended half-life concentrates were recently introduced and limited data have shown extended terminal half-life (THL). However, real-life data on pharmacokinetics in large cohorts of patients with haemophilia (PWH) and information on the effects of age, body composition and blood group (THL) are lacking.
Aim: to assess THL for standard half-life (SHL) and extended half-life (EHL) concentrates according to age and blood group.
Methods: Data on THL, age and blood group were extracted from the WAPPS (Web-Accessible Population Pharmacokinetics Service; www.wapps-hemo.org) database. WAPPS provides an on-line service of individual pharmacokinetic (PK) calculations for clinicians, based on concentrate-specific population pharmacokinetic models. Informed consent was waived by the ethical committee. THL according to age and blood group was assessed by multivariable linear modelling.
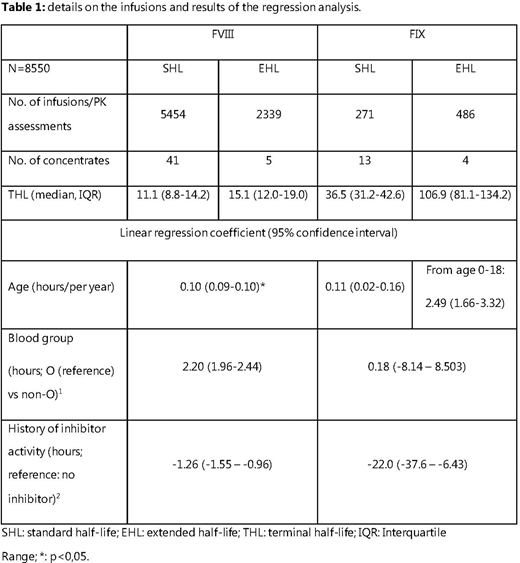
Results: Infusion data (n=8550) was collected from 4832 (2222 children, 2610 adults) patients with severe haemophilia (89% haemophilia A; 34% treated with EHL concentrates, 9.7% with history of inhibitors, median age: 20 (range: 1 month - 85 years)). Details on infusions, calculated THL and results from regression models are shown in Table 1.
For factor VIII, median THL was longer in EHL at 15.1 hours (interquartile range (IQR): 12.0-19.0) vs. 11.1 hours (8.8-14.2) in SHL-FVIII. Linear models identified age, type of concentrate and blood group as independent predictors of THL in FVIII. THL increased with age by 1 hour/10 years, and THL was 2.2 hours longer in patients with blood group non-O, independent of concentrate type.
For FIX, median THL was considerably longer in EHL at median 106.9 (81.1-134.2) vs. 36.5 (31.2-42.6) hours in SHL. Age was only a significant predictor of THL in children using EHL-FIX concentrates: THL increased by 2,5 hours/year until adulthood for EHL concentrates (e.g.: from 77 hrs at age 4 to 112 hrs at age 18), whereas THL was stable across all ages for SHL-FIX. THL was stable across blood groups for all FIX concentrates.
In PWH with a positive inhibitor history, THL was decreased by 1,3 hours for FVIII and 22 hours for FIX.
Discussion: This study was the largest study describing THL according to concentrate type and patient characteristics so far. At group level, a significant extension of THL was confirmed for both FVIII-EHL and FIX-EHL. THL was associated with age and blood group for all FVIII concentrates. In contrast, THL for FIX concentrates, was only associated with age in children using EHL-FIX. THL was significantly reduced in patients with a history of inhibitors. The results support the need for individual assessment of THL, especially for patients with haemophilia A and children treated with EHL-FIX.
This was a group-based study. Within the age of personalized medicine, individualized PK assessments seem more appropriate. Our next project will be to analyse the effects of switching from SHL to EHL in individual patients.
Versloot:Bayer: Research Funding. Germini:Bayer: Research Funding; Roche: Research Funding; Takeda: Research Funding; NovoNordisk: Research Funding. Iorio:CSL: Research Funding; BioMarin: Research Funding; Bayer: Research Funding; Uniqure: Research Funding; Takeda: Research Funding; Spark: Research Funding; Sanofi: Research Funding; Roche: Research Funding; Pfizer: Research Funding; Octapharma: Research Funding; NovoNordisk: Research Funding; Grifols: Research Funding; Freeline: Research Funding. Fischer:Bayer, Biogen, Pfizer, Baxter/Shire, and Novo Nordisk: Research Funding; Bayer, Baxter, Biogen, CSL Behring, Freeline, Novo Nordisk, Pfizer, Roche, and Sobi: Consultancy; Bayer, Baxter/Shire, SOBI/Biogen, CSL Behring, Octapharma, Pfizer, NovoNordisk: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal